Innovative HER2 CTC Assay in Development
Enhance longitudinal monitoring in breast cancer, predict therapeutic responses, and optimise subject selection for clinical trials with ANGLE’s advanced HER2 testing. Leveraging ANGLE’s Parsortix® platform for circulating tumour cell (CTC) harvesting from a single blood sample, researchers can measure HER2 protein expression via immunofluorescence (IF) staining and quantify HER2/neu gene amplification with fluorescence in situ hybridisation (FISH).
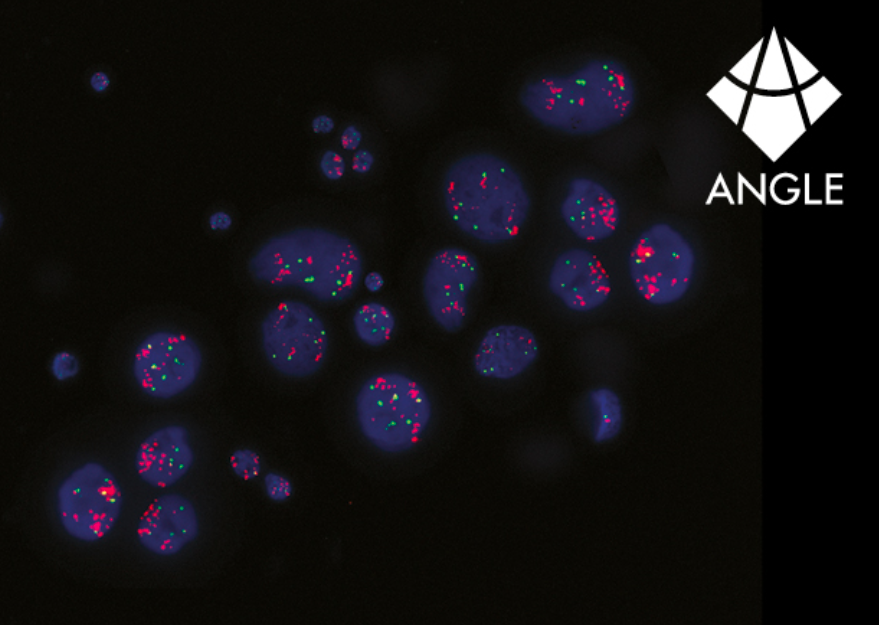
ANGLE is developing an end-to-end assay for the evaluation of human epidermal growth factor receptor 2 (HER2) gene amplification and protein expression in circulating tumour cells (CTCs) harvested using the Parsortix system from the blood of metastatic breast cancer (MBC) patients. ANGLE’s in-house study reports the successful identification of the HER2 protein and gene copy information from cell targets with minimal cell loss. The analysis of clinical samples using the assay has demonstrated the ability to detect HER2 positive CTCs in patients who were previously HER2 negative based on a primary tissue biopsy. This assay is being developed as a product kit for minimally invasive identification of HER2 positive and HER2 low patients, who may benefit from HER2-targeted therapies and HER2 antibody-drug conjugates.
The combination of ANGLE’s Parsortix system for harvesting CTCs for analysis, BioView’s imaging and analysis technologies, and the new HER2 CTC staining kit under development is intended to provide an easy to use assay based on a simple blood test, providing an up-to-date HER2 status for breast cancer patients on a repeat basis.